Listeria Management of Ready-to-eat Meat Products PrimeSafe Technical Guideline
Learn how to manage Listeria effectively when processing ready to eat meat products.
Summary
- This is a guideline only. Be sure to understand the standards referred to by this guideline.
- You must have a licence and a Listeria management plan to process and package ready-to-eat (RTE) meat and poultry products.
- Use this guideline to understand how to manage listeria and what to cover in your Listeria management plan.
Requirements to manage listeria
- All facilities processing and packaging Ready to Eat (RTE) meat products in Victoria must have a Listeria Management Plan and be licensed by PrimeSafe.
- Raw meat products must be segregated from RTE products.
- RTE products that are cooked, must have a core temperature of at least 65°C for 10 minutes. All processing data must be recorded and stored for future reference and any alternative heating processes must be validated and approved by PrimeSafe.
- All meat products must be cooled in compliance with AS 4696:2023 and during the cooling of RTE products any risk of contamination must be minimised. Alternative cooling processes must be validated and approved by PrimeSafe.
- A minimum of five environmental sites must be sampled for Listeria monthly and any RTE product must be tested quarterly to verify procedures.
- When first commencing RTE operations, Listeria monocytogenes (Lm) must be tested fortnightly for the first 3 months and any new RTE products must be validated for Listeria monocytogenes. If no positive results are found then quarterly testing plan can commence.
- If there is an interruption to processing, then a representative batch must be processed quarterly and tested.
- Seasonal sampling programs can be implemented at facility’s that only produce packaged RTE meat products infrequently.
- A minimum of one 25-gram final product sample must be tested for each product type.
- Samples must be tested using approved methodology by a NATA certified laboratory and any alternative methods must be validated and approved by PrimeSafe.
- If environmental swabs or RTE product test positive for Listeria monocytogenes then PrimeSafe must be notified within 24 hours, product isolated and placed on hold. Any product that has left the premises must be recalled. A clearance program of test and hold undertaken until acceptable results are obtained for three consecutive batches (a batch is considered as up to 24 hours of continuous production or a lesser period between cleaning and sanitation procedures).
Listeria is a food borne pathogen found frequently in raw foods. It spreads to processed foods through cross contamination, poor hygiene and inadequate processing. The most common foods associated with Listeria include dairy, meat, seafood, fruits and vegetables. Listeria has created significant problems for consumers and the food industry, including serious illnesses, product recalls and short-term plant closures. The growth of Listeria is enhanced in processed “Ready to Eat” (RTE) products, especially those packed under vacuum or modified atmosphere (MA) using nitrogen gas. RTE foods are at risk of being contaminated prior to packaging as they may not always be cooked or reheated prior to consumption.
There are a few different types of Listeria but the most common one associated with food borne illness is Listeria monocytogenes (LM) that can cause serious illness or even death in vulnerable people such as the very young, elderly, pregnant or immunocompromised individuals. The related food borne illness “Listeriosis” can display a range of symptoms including fever, chills, convulsions, backache, headache, gastroenteritis, diarrhoea, vomiting and in extreme cases meningitis, septicaemia and spontaneous abortion.
Listeria survival and growth
- It can occur at temperatures as low as 1°C and survives the freeze-thaw process.
- It is unlikely to grow below pH 4.6, with ideal growth conditions in the pH range 5.0 – 9.0.
- It continues in dry conditions with low water activity of 0.90 (e.g. dry soil, straw, faeces and hides of animals).
- It can occur in salt solutions of up to 10% (e.g. pre-prepared brine solutions).
- It can survive without oxygen and can be found in vacuum packed and modified atmosphere products.
- It is destroyed by cooking or heating to a core temperature of at least 65°C for 10 minutes or equivalent.
All facilities processing and packaging RTE meat products in Victoria must be licensed by PrimeSafe and have a Listeria Management Plan in place as part of the Food Safety Program. The plan must ensure that raw products are segregated at all times from cooked RTE products. Listeria as a food safety hazard can be managed collectively using two key indicators:
- Environmental Monitoring.
- Product Testing.
The Listeria Management Plan must include the following:
- Identification of the trained personnel who will be responsible for the plan (e.g. QA Manager).
- Schedule and records for training of samplers and key production personnel.
- A sampling plan showing each area where environmental and product samples are collected.
- Procedures for collecting environmental and product samples.
- Testing arrangements – NATA certified laboratory or in-house testing.
- Record keeping of all test results.
- The response taken when Listeria is found.
- A review of the plan annually or when there is a change in manufacturing processes.
Sampling sites across the processing environment must be selected based on a risk assessment. All possible contamination sites should be included and be specific to the processing operations of the business. For areas where RTE products are manufactured, swabbing should be conducted at a minimum of 5 times each month before and during processing operations. Testing must be done using approved methodology and by NATA registered laboratories. Any in-house testing must be done using methodology approved by independent bodies such as The Association of Official Analytical Chemists (AOAC). If test kits are used, then verification (proficiency testing) must occur annually to validate the kit methodology compared to a laboratory-based methodology. For ease of testing, sampling sites can be divided into two zones.
| Description | Examples of sampling sites within processing areas |
| Zone 1 – Food Contact | Slicers, dicers, hoppers, conveyors, spiral freezers in direct contact with cooked product, tables, benches, utensils, open bearings on machines, brine tanks. |
| Zone 2 – Non Food Contact | Floors, walls, ceiling, drain outlets, condensate in chillers and freezers, conveyors, air vents, chiller doors, switches, floor joints and crevices, pooling water. |
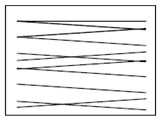
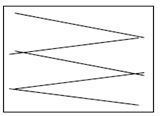
Swabbing must be done using sterile implements such as cotton buds, sponges or gauzes. For a moist surface area use a dry swab and for a dry surface area use a swab moistened with sterile peptone water. The swab must be taken following a zigzag pattern, close together covering at least 50cm2 as shown below. If using a cotton bud it should be rotated to cover the whole swab. Swabs must be hygienically stored at temperatures below 5°C and tested within 24 hours of sampling.
Correct swabbing technique
Incorrect swabbing technique
Ready to Eat Meat Products Listeria testing requirements
FSANZ Standard 1.6.1 states the following limits for Listeria Monocytogenes in RTE food products:
| Food Category | Limit |
| Food supporting growth of L. monocytogenes | Not detected in 25 g |
| Food not supporting growth of L. monocytogenes | ≤ 100 cfu/g |
Ready to Eat Food Not Supporting the Growth of Listeria
A RTE food is considered as not supporting the growth of LM under FSANZ Standard 1.6.1 if any one of the following parameters applies:
(i). Refrigerated shelf life ≤ 5 days;
(ii). pH < 4.4;
(iii). Water activity < 0.92;
(iv). pH < 5.0 in combination with a water activity < 0.94;
(v). Frozen product (consumed frozen or thawed immediately before consumption); or
(vi). Validated data showing L. monocytogenes will not increase by >0.5 log cfu/g over the stated shelf life.
These parameters allow the product to be classified as “not supporting the growth of LM”.
Sampling Sizes and Frequency
All product types (i.e. whole products, portioned products, sliced products, cooked sausages, etc.) produced at a facility must have one representative product unit sampled for LM at the frequency prescribed in Australian Meat Regulators Group (AMRG) – Standard 4.2.3: Guidelines for the Management of Listeria (June 2019). Sampling must reflect normal production conditions and include all associated processing categories produced onsite.
The facility may collect a representative sample of a product for LM testing in-house to send to a laboratory, rather than sending a whole unopened unit. When doing so, staff must use strict aseptic techniques during sampling and handling to prevent cross-contamination. A positive LM result from a representative sample is treated the same as a detection in an unopened packaged product and will trigger a full investigation, corrective action, and verification requirements as per the AMRG Standard 4.2.3 requirements.
Unpackaged smallgoods with a shelf life under 5 days are not required to comply with the requirements of the AMRG Standard 4.2.3.
Product Sampling Example
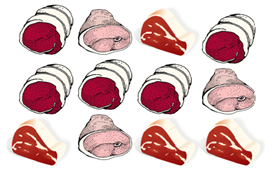
Figure 1: Take one 25-gram sample from each product type (i.e. whole, portioned, sliced, etc).
Seasonal Listeria Testing
Some typically smaller facilities only undertake seasonal smallgoods production yet are required to test RTE packaged products for LM at least quarterly and undertake monthly environmental swabbing throughout the year under the AMRG Standard 4.2.3. This is largely to ensure that potential contamination incidents are identified early rather than waiting for this to occur at peak production periods (i.e. festive seasons). This can be overcome through requiring early intensified LM sampling of the environmental in the lead-up to recommencing production and through requiring these manufacturers to undertake test and hold arrangements.
A facility intending to conduct only seasonal smallgoods production of packaged RTE products must have a documented Listeria Management Plan in their Food Safety Program specifying that they are a seasonal packing facility of RTE products. The procedures can be approved by the facility’s auditor if they state that the facility:
(i). Conducts environmental swabbing over a minimum of 2 weeks, prior to commencing production, that includes all RTE production areas showing these areas are free of LM; and
(ii). Produces packaged RTE products on a test and hold basis throughout the seasonal production period.
Any business without approved procedures to conduct seasonal testing must otherwise comply the AMRG guidelines and undertake quarterly product testing and monthly environmental swabbing.
Evidence to Support Applications
PrimeSafe accepts applications from licensees for requests for alternative compliance to the applicable standards, such as claims that a product may not support the growth of LM. PrimeSafe implements the FSANZ Guidance on the application of microbiological criteria for LM in RTE food and allows licensees to use these criteria.
A business may also produce a predictive model which will be considered in support of an application.
Challenge studies may also be considered by a business to support an application, but these are not mandatory.
Approximate Timelines for Listeria monocytogenes Testing of RTE Meat Products
| Timeline | Business Responsibility | Testing Laboratory Responsibility |
| Step 1 | Submit five chilled packaged, unopened samples as it would be sold. | Ensure received packaged samples are chilled unopened. Test all five samples submitted. |
| Step 2 | Trained person(s) receiving the information review procedure and require other staff to ensure GMP and hygiene related procedures are repeated. | Laboratory suspects Listeria species from presumptive positive. Inform the business. |
| Step 3 | Review the result and decide what actions to take. Product must be placed on hold and improve GMP practices. PrimeSafe must be informed. | Laboratory confirms that Listeria species is present but not necessarily L. monocytogenes. Inform the business. |
| Step 4 | Withdrawal of product, undertake corrective actions as advised by Department of Health and PrimeSafe. | Confirmed L. monoctyogenes. Advise the Department of Health and the business. |
For further information
Cox B and Bauler M (2008), Cook Chill for Foodservice and Manufacturing: Guidelines for Safe Production, Storage and Distribution; AIFST.
Food Microbiology Group, AIFST Inc. NSW Branch (2003), Sixth Edition.
Food Standards Australia New Zealand, Food Standards Code (www.foodstandards.gov.au)
PrimeSafe, AMRG Guidelines for the Management of Listeria (www.primesafe.vic.gov.au)
Disclaimer
This information is a guide only and must not be used in place of the current Standard. PrimeSafe does not guarantee its accuracy, reliability, currency or completeness, therefore it cannot be used to substitute for legal or professional advice. PrimeSafe accepts no legal liability arising from reliance on any part of this document.