Ageing of Beef PrimeSafe Technical Guideline
This technical guideline explains ageing of beef processes and how to get approval from PrimeSafe to age beef.
Summary
- This is a guideline only. Be sure to understand the standards referred to by this guideline.
- The guideline describes dry and wet ageing processes and how they affect meat quality and safety.
- Find out how to get approval to age beef and how to test your product.
Fresh unpackaged carcass and meat can be stored providing it remains wholesome, as indicated by the absence of dryness, discoloration, visible mould growth, sliminess or odour.
Meat stored for ageing purposes must be subjected to a controlled, documented and approved ageing procedure.
Ageing is the process during which microbes and enzymes act upon the meat to help breakdown the connective tissue to tenderise the meat. There are two ways ageing can be accomplished:
- Wet ageing by placing beef in a plastic bag under vacuum; or
- Dry ageing by storing beef in a temperature and humidity-controlled environment.
The main difference is that wet ageing results in little or no moisture loss, whereas dry ageing can result in up to 50% moisture loss. Product labelling should indicate the ageing processes used.
The quality of meat prior to ageing is critical. If meat of inferior microbiological quality is used, pathogens can grow quickly and produce harmful toxins that may not be destroyed during cooking. The microbiological quality of aged meat should be monitored by testing for pathogenic as well as spoilage bacteria. The microorganisms that must be tested by an approved laboratory are E. coli and Enterobacteriaceae. These are indicative of other pathogens that are likely to grow. Enterobacteriaceae is a group of bacteria including Salmonella, Shigella and Yersinia ranging in different pathogenicity.
Wholesomeness of meat
The quality and wholesomeness of meat for human consumption can be determined by basic observations of dryness, discoloration, visible mould growth, sliminess and odour. Meat processing facilities are required to comply with the requirements of Australian Standard for the Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (AS 4696) with regards to the management of wholesomeness.
When ageing meat for human consumption, licensees are required to have the following approved procedures in place:
- A documented procedure that covers the ageing process.
- Controls to ensure aged meat does not leave the premises unless it is wholesome and accurately defined in accordance with the relevant standard.
- Controls to ensure aged meat not fit for human consumption is removed from the food chain and dealt with separately to other meat products.
- Specifies how the aged meat has met the requirements of the relevant standard.
- Implementation of a HACCP plan for each stage of production of aged meat.
Approval requirements
1. All PrimeSafe licensed businesses intending to manufacture dry aged beef for human consumption must:
- Comply with the requirements of the Australian Standard for the Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (AS 4696);
- Inform PrimeSafe of their intent to commence producing dry aged meat; and
- Provide proposed manufacturing procedures in their amended Food Safety Program for approval before starting to dry age beef.
2. If the Accepted Measures defined in this guideline are used, approval of a revised Food Safety Program for dry ageing of beef can be considered by the licensee’s approved Conformity Assessment Body (CAB) auditor.
3. If variation to the Accepted Measures in this guideline are proposed, the amended Food Safety Program must be submitted directly to PrimeSafe.
4. The following requirements must be considered in their application:
- Changes to premises and the installation of equipment that complies with AS 4696 and the ability to facilitate the control of temperature, humidity and air velocity.
- Changes to the Food Safety Program including an amended HACCP plan.
- Documented procedures and validation records.
- Segregation of dry aged meat from other meat products.
- Accepted Measures of the ageing process.
- The intended duration of the ageing period and testing of mould one week prior to completion of the ageing period.
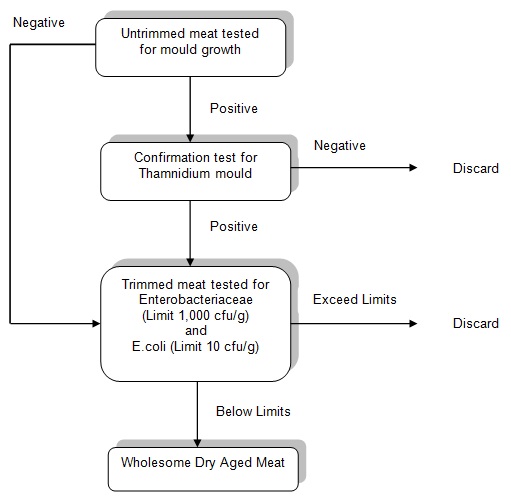
- Validation of the process by mould testing, if there is mould growth, then confirmation that it is Thamnidium mould.
- Confirmation of product wholesomeness by shelf-life testing of trimmed meat for Enterobacteriaceae and E. coli at the end of the ageing period.
Accepted Measures
1. Premises and equipment must meet the requirements of the Australian Standard for the Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (AS 4696) and be approved by a PrimeSafe inspector.
2. Dry ageing storage conditions:
- Temperature: between – 0.5°C to 1°C (2°C to 3°C) may be used when only ageing for up to 3 weeks).
- Relative Humidity: between 75% to 85%.
- Air velocity: between 0.2 to 0.5 m/s.
3. Wet ageing storage conditions:
- Storage below 5°C;
- Validation testing that any mould is Thamnidium;
- Shelf-life testing for Enterobacteriaceae and E. coli at the end of the ageing period.
Dry ageing involves the degradation of connective tissue and muscle protein structure of the meat of carcasses or cuts of meat and must be managed to ensure growth of beneficial and non-harmful moulds. Best practice is to reduce carcasses to smaller primals and sub-primals in preparation for the ageing process. The most popular cuts used for ageing are strip loin, rib eye and sirloin that can be aged in dedicated refrigeration units or rooms. For dry aged meat, the fat cap is often left on the meat to help with flavour development, retention of moisture and reduction of trim loss when the crust is eventually trimmed off.
Dry aged beef has an intense flavour when compared to wet aged meat that can have a sour blood/serum flavour. The reason for this is that the predominant bacteria on dry aged meat are the Pseudomonas that grow in the presence of oxygen. This is different to wet aged packaged meat where Lactobacilli bacteria grow in the absence of oxygen. The Lactobacilli bacteria convert lactose to lactic acid therefore wet aged product may have a slightly sour taste or odour when compared to the Pseudomonas that do not produce any sour flavours on dry aged meat.
Dry aged beef is considered a gastronomical treat and is commonly found in some of the finest restaurants and butcher shops. Premium dry aged beef products usually come from grain fed cattle due to the greater marbling within the meat. Extremely lean meat will spoil if aged. The flavour of dry aged beef can range from buttery to nutty and almost gamey depending on the age and storage conditions. The flavour is also dependent on other factors such as the quality of the meat cut, whether it is grass fed or grain fed, storage temperature and relative humidity during ageing. Premium products can be dry aged for up to 6 weeks provided the process meets the requirements of these guidelines. It may be difficult to maintain the wholesome of meat after 8 weeks of dry ageing.
The Processes
- Enzymatic action
Endogenous proteolytic enzymes from the meat itself as well as from the specific beneficial moulds weaken the structural myofibrillar proteins in the meat. This takes 10 to 14 days and results in a more tender meat. At this stage the meat flavour can usually be described as buttery and smooth.
- Evaporation
Loss of water from the meat by evaporation causes concentration of the remaining proteins and increases flavour intensity to a nutty almost gamey taste. Final water loss can be up to 50 per cent and depends on the relative humidity used during drying.
Control Factors
- Temperature
A storage temperature between -0.5°C to 1°C should be used. A temperature of between 2°C to 3°C may be used when the meat is only aged for 2 to 3 weeks. Frozen or thawed meat must not be aged because the desired enzymatic action will not occur, and mould growth will not be initiated on the surface of the meat. The temperature must be recorded daily throughout the ageing process to ensure the wholesomeness of the meat is maintained in compliance with the requirements of Australian Standard for the Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (AS 4696:2007).
- Relative Humidity (RH)
Control of RH is important because it restricts growth of pathogenic bacteria by drying the meat surface resulting in the formation of a crust. This also reduces bacterial growth on the surface in preparation for growth of the desirable Thamnidium mould. A RH of between 75% to 85% is recommended and actual RH should be recorded daily for the duration of the ageing process. Lower RH may be used but tends to dry out the meat and contribute to higher trim losses in the final product. Higher RH should not be used because it will result in spoilage of the meat before ageing is complete.
- Air Flow
To prevent spoilage, portions of meat must be adequately separated from each other to allow efficient and controlled air flow between each portion. The desirable air velocity is 0.2 to 0.5 m/s and can be controlled with a properly designed refrigeration unit and fans. The air velocity and flow should be kept uniform for the duration of the drying process and is most critical at the start of the dry ageing process.
- Cross contamination
Dry aged meat must be segregated from all other meat products. Dry ageing must not be conducted in chillers where other fresh meat is stored. Purpose built rooms and cabinets must be used for the dry ageing of meat. Trimming and preparation of product for packaging and sale must be segregated from areas used for fresh meat. Dry aged meat products must not be displayed in retail display cabinets with other fresh meat. Designated cabinets and/or chillers must be used.
- Antibacterial Strategies
The use of ultraviolet (UV) light for destruction of bacterial cells is well known for fresh meat. A more sophisticated approach to manage the dry ageing process is to install UV lighting entirely and leaving no other light source. Air can also be circulated through UV lit chambers however the costs may be prohibitive. The use of antibacterial rinses for the preparation of meat for dry ageing has some inherent risks and must be validated and approved.
- Microbiological
Dry ageing involves restricting bacterial growth and encouraging the growth of beneficial mould. During the dry ageing process, mould from the Thamnidium, Penicillium, Rhizopus and Mucor genera can be found on the surface of the meat. The most desirable is the Thamnidium mould as it has been shown to releases proteases that tenderise ageing meat. Other mould species have been associated with infections in humans and production of harmful natural toxicants. They also do not provide any favourable characteristics for ageing of meat.
Wet ageing of meat describes a general storage process that occurs after a carcass is broken down or boned. The wet ageing process commences when meat is packaged and stored. If vacuum packed meat is aged, then it must be validated as being wholesome and fit for human consumption (see Shelf Life and Labelling Requirements for Meat Products PrimeSafe Technical Guideline).
The process
1. Enzymatic action
Endogenous proteolytic enzymes weaken the structural myofibrillar proteins in the meat. This takes 10 to 14 days and results in a more tender meat. Meat is aged in its own blood and serum therefore growth of Lactobacilli can result in a sour and subtle flavour compared to dry aged meat.
Control factor
2. Temperature
A storage temperature below 5°C must be used. Frozen or thawed meat must not be aged because the desired enzymatic action will not occur, and mould growth will not be initiated on the surface of the meat. The temperature must be recorded as required in the food safety program throughout the ageing process to ensure the wholesomeness of the meat is maintained in compliance with the requirements of Australian Standard for the Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (AS 4696).
Product testing is required for:
- Initial approval and annual validation.
- Any change to the process.
The dry ageing of beef can be for up to eight weeks, depending on the product characteristics required. The ageing conditions will determine the shelf life of the final product. Dry aged meat products must be tested for mould to validate the procedure. Growth of Thamnidium mould can start from three weeks after commencing the ageing process. Testing involves removing a 100g portion of untrimmed aged meat that includes visible mould if it is present and sending it for testing at a NATA accredited laboratory. If testing for mould shows that the results are positive, then confirmation that the mould is Thamnidium must be conducted.
For wet aged meat products there will be no growth of mould due to the lack of oxygen in the bag therefore there is only a requirement to confirm the wholesomeness of the product.
Processed (trimmed) and packaged dry aged meat cuts generally have a shelf life of two to three days. For this reason, meat is trimmed just before sale. To confirm the wholesomeness of dry and wet aged products, the shelf life must be validated by testing for Enterobacteriaceae and E. coli. These two tests must be conducted at the end of the desired shelf life following the ageing period to validate the wholesomeness of the final product at the point of sale. The critical limits for wholesomeness for these purposes are microbiological limits of Enterobacteriaceae of 1,000cfu/g and E. coli of 10cfu/g. Five 10-gram samples must be taken for testing of both Enterobacteriaceae and E. coli. Composite testing of the samples is permitted at the laboratory.
All procedures and records must be submitted to PrimeSafe before approval of your process may be granted.
Testing flow
Further Information
Dashdorj D., Tripathi V.K., Cho S., Kim Y. and Hwang Inho; Dry Aging of Beef; Review. Journal of Animal Science and Technology (2016) 58:20.
Jensen L. B. (1944), Microbiological Problems in the Preservation of Meats, Research Laboratories, Swift & Company, Chicago, Illinois.
Dillion, V. M. (1998). Yeasts and moulds associated with meat and meat products. The Microbiology of Meat and Poultry, 85-110.
Disclaimer
This information is a guide only and must not be used in place of the current Standard. PrimeSafe does not guarantee its accuracy, reliability, currency or completeness, therefore it cannot be used to substitute for legal or professional advice. PrimeSafe accepts no legal liability arising from reliance on any part of this document.